KeTMo
Title
Low-dose KETamine as an adjunct to MOrphine for acute pain in the ED: a randomized, double-blinded, trial: KeTMo
Aim
To improve acute pain treatment in the ED, for both opioid tolerant and opioid naïve patients.
Methods
This is an investigator-initiated, randomized, parallel-grouped, double-blinded, superiority trial, investigating the combination of IV LDK and IV morphine versus IV morphine and placebo as regards to analgesic effect.
160 patients admitted to the ED with acute pain (NRS ≥5) will be enrolled, 80 with a prior use of opioids and 80 without a prior use of opioids.
Included patients will be randomized in a 1:1 ratio to either low dose ketamine or placebo as an adjunct to morphine.
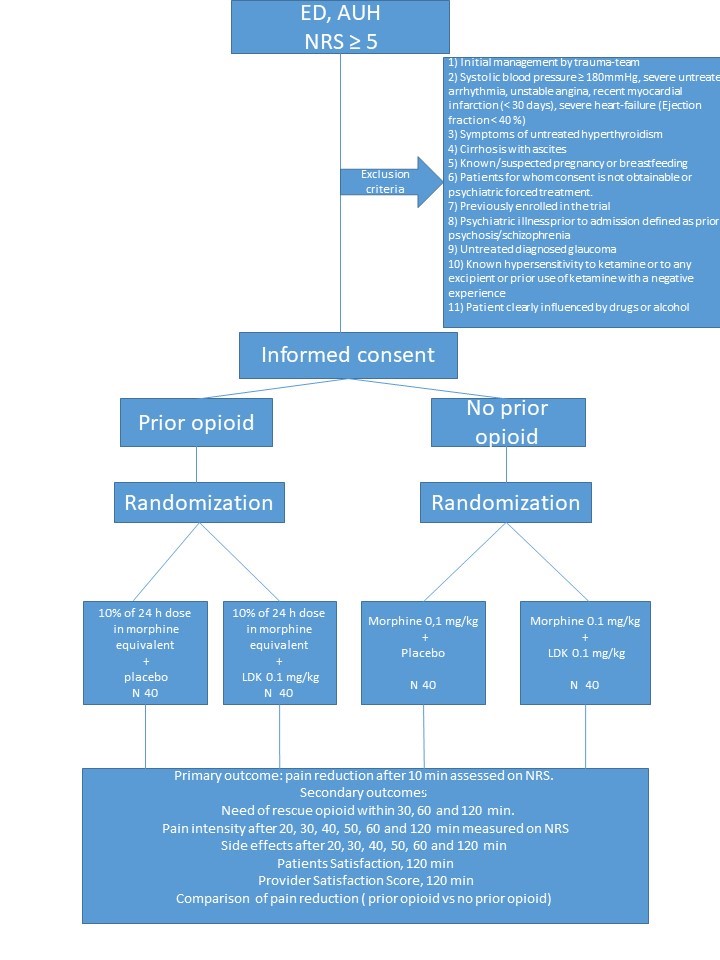
Population
Adults, ≥ 18 years, admitted to ED with a pain full condition, NRS ≥5.
Perspectives
This is the first study to examine the effect of LDK as an adjunct to morphine in a general patient population in the ED with a prior use of opioids and to compare it with the effect for patients without a prior use of opioids.
This study could present a better pain treatment for patients with and without a prior use of opioids.
Primary investigator
Stine Fjendbo Galili
Sponsor
Professor Lone Nikolajsen
Registration numbers
EudraCT: 2021-005116-64 VEK: 1-10-72-334--21 LMST: 2021100973
Funder
Health Research Foundation of Central Denmark Region





